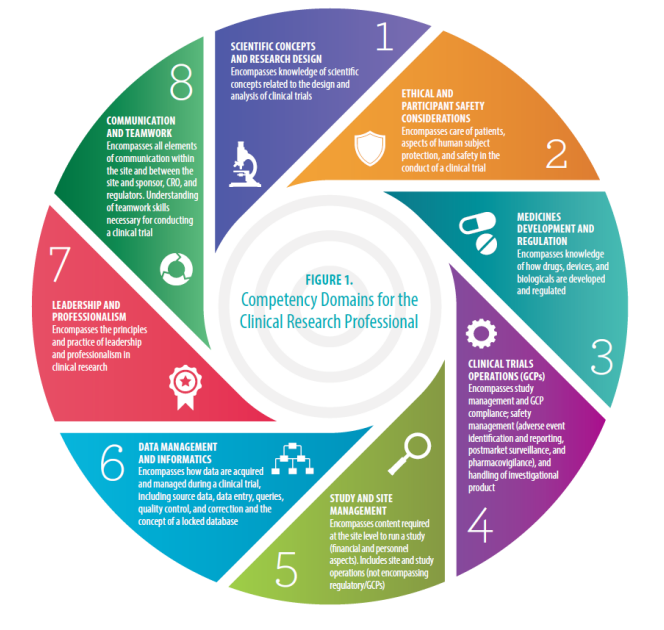
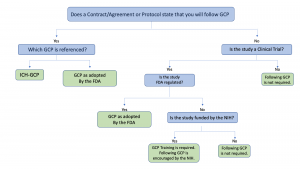
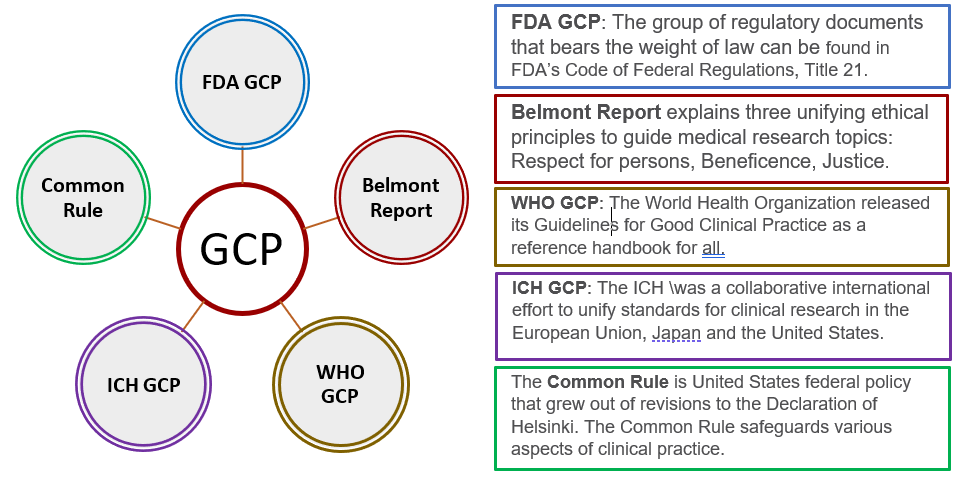
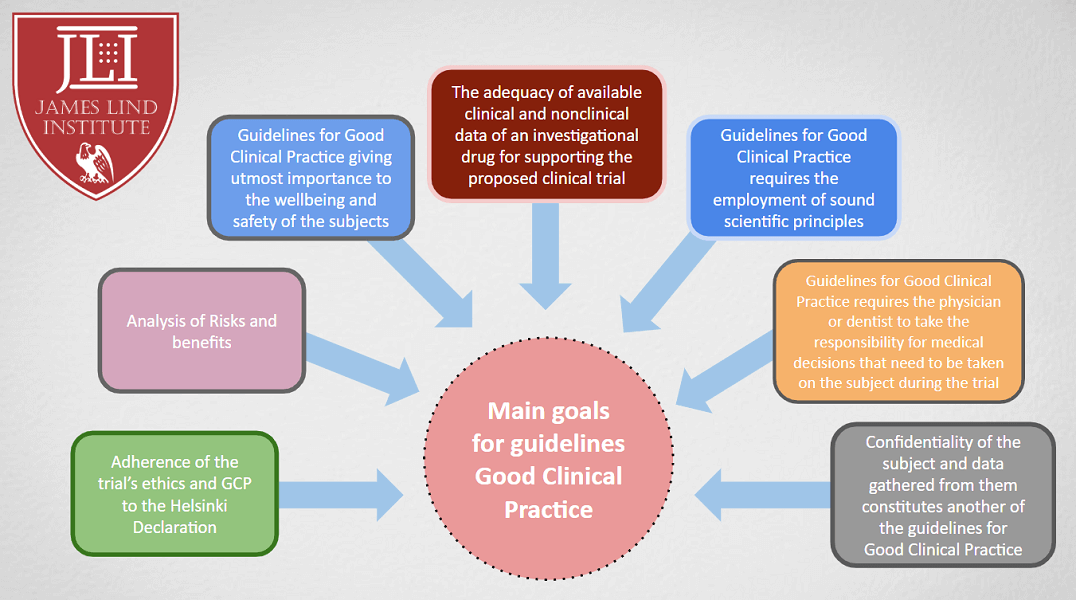
Good Clinical Practice (GCP) Training and Finding | Good Clinical Practice ( GCP) Training and Finding

EU clinical research framework. ICH GCP = International Conference on... | Download Scientific Diagram
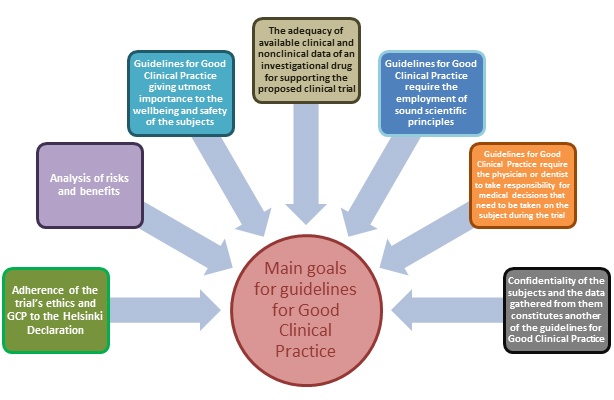
The Good Clinical Practice (GCP) and the responsibilities of pharma sponsors - Avantyo article in Viata Medicala magazine · News · Avantyo

GCP, Good Clinical Practice Acronym, Business Concept Background Stock Photo, Picture And Royalty Free Image. Image 140132000.

REFRESHER: ICH Good Clinical Practice (GCP) E6 (R2) and regulatory requirements for Clinical Trials (Fiona Stanley Hospital) - RETProgram

The Good Clinical Practice (GCP) and the responsibilities of pharma sponsors - Avantyo article in Viata Medicala magazine · News · Avantyo

ICH GCP - 8. Essential documents for the conduct of a clinical trial: ICH E6 (R2) Good clinical practice

AO Foundation в Twitter: „The AO's Good Clinical Practice (GCP) course for surgeons takes place November 23-24, in Dübendorf, Switzerland. Register now! https://t.co/uRZ5yXndRU #AOFoundation #AOPEER #research #education https://t.co/gIgbFXwkci“ / Twitter