PDF) Clinical study reports of randomised controlled trials: An exploratory review of previously confidential industry reports

Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI Extension | The BMJ
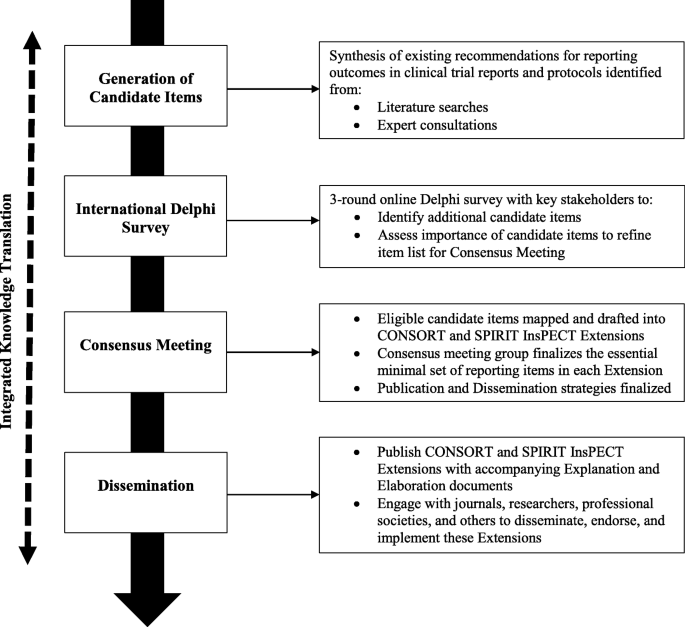
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text
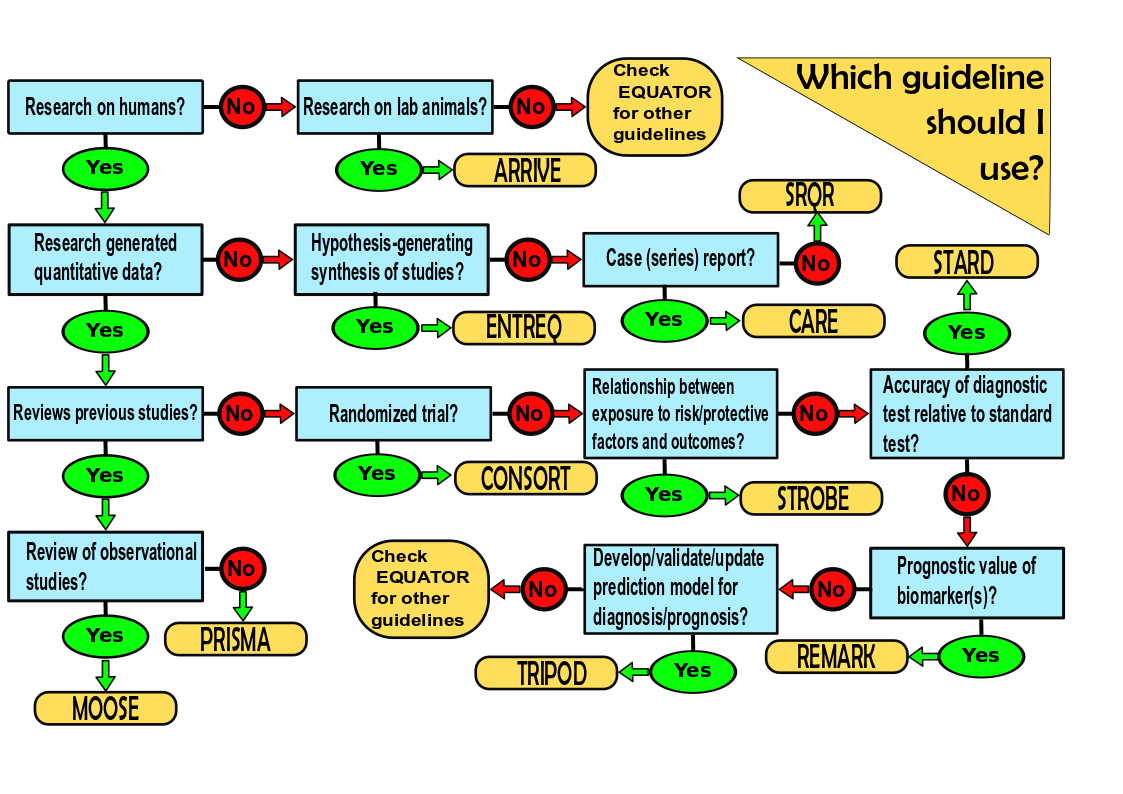
Biomedical study reporting guidelines - Essential guides to writing high-quality scientific reports - SciMeditor
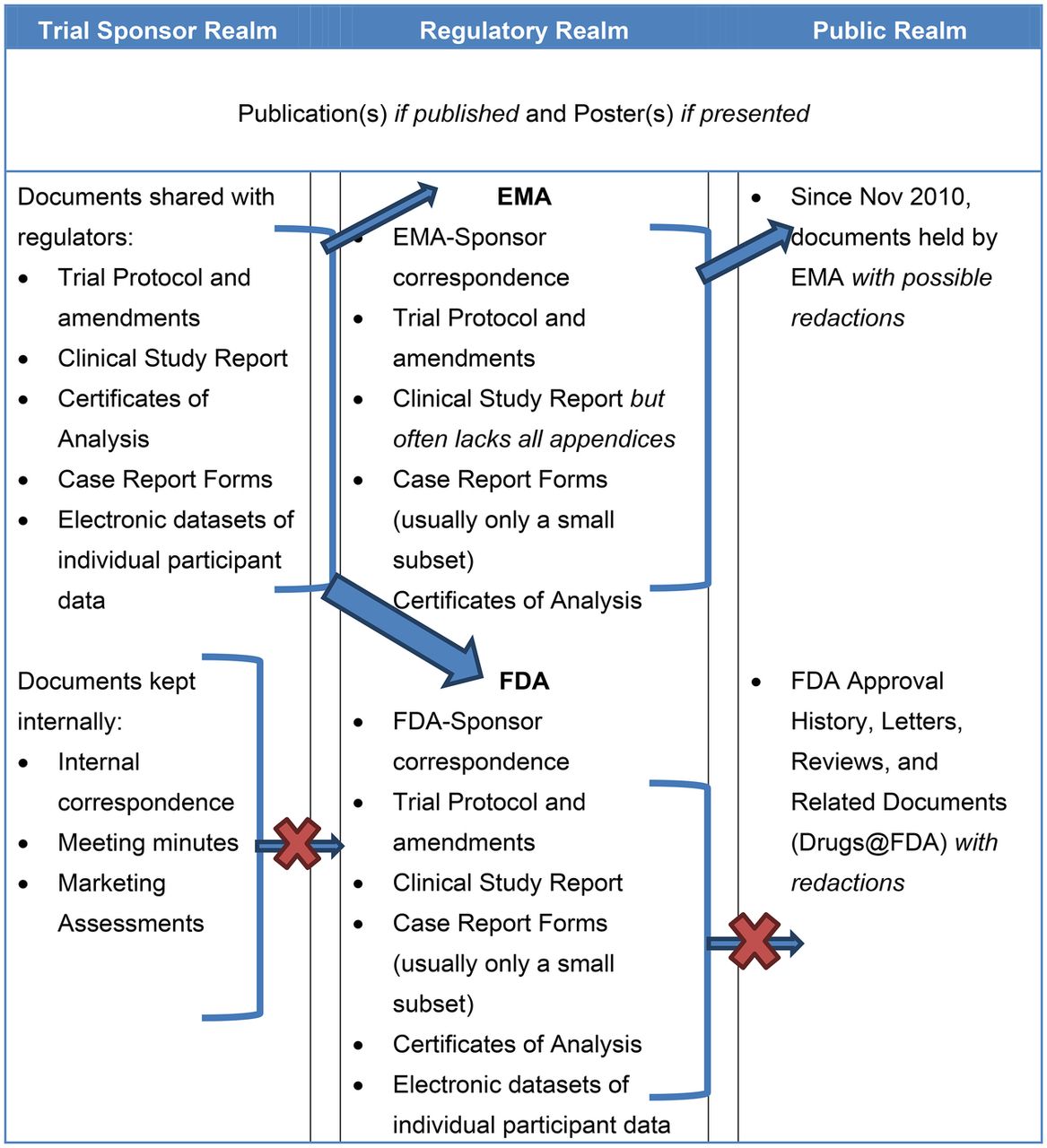
Clinical study reports of randomised controlled trials: an exploratory review of previously confidential industry reports | BMJ Open